UC Riverside Take-Out Bee Traps
by P. Kirk Visscher
Department of EntomologyUniversity of California
Riverside, California 92521
Abstract
"Straggler" honey bees remaining behind after removal of a swarm are attracted by synthetic bee pheromones. Queen mandibular pheromone is more attractive than Nasonov gland pheromone, and a mixture of both pheromone blends is even more attractive. A trap using these baits is simple to produce, fairly inexpensive, easy to deploy in a variety of situations, and effective in containing and killing straggler bees.
Introduction
The immigration of Africanized bees into the United States, and widespread fear of these more defensive bees has increased the public's demand for removal of swarms and feral colonies of bees. At the same time it has made bee removals more problematic. Compared to European bees, Africanized bee colonies swarm more frequently and occur at higher density, so that swarms are more frequently encountered. When swarms or colonies are discovered, there is an increased likelihood that the bees will sting, and a much increased perception that they are dangerous. At the same time, there are fewer beekeepers willing or permitted to perform bee removals. Pest control specialists, and in some areas public agencies, are removing increasing numbers of bee colonies.
Swarms may be removed by hiving them or by spraying them with insecticides, including soap or detergent solutions (Sames et al 1991; Visscher et al. 1995). A major problem with either technique is that "straggler" bees remain at large after most of the bees have been killed or hived. Most of these are bees that were scouting nest sites or foraging away from the swarm cluster when control efforts began. Seeley et al. (1979) estimated that about 5% of the bees in a swarm participated in scouting one nest site, so in swarm of 10,000 or more bees, the scouts can comprise several hundred bees. Straggler bees from a removed swarm remain near the swarm site, but fly around a great deal searching for their queen. These bees can survive for at least several days, and are likely to run short of food, which may make them more likely to sting.
The problem of straggler bees can be solved by transporting the hive only at night after the bees have settled, by spraying them at night when all the bees are likely to be on the swarm cluster, or by returning to the site at night, locating the clustered stragglers and repeating the insecticide application. These solutions are unsatisfactory because the swarm or straggler bees remain a threat for a longer time, and they require persons involved in swarm abatement to work outside of normal hours, and to return to the site.
We had two objectives in the research we report here. The first was to develop methods that would reduce irritability and flight of straggler bees between the initial control and a return visit. This could be done by reducing the flight activity near the site from which a swarm was removed, and by making it possible to control exactly where the bees cluster; this would also facilitate control on a return visit, especially in cases in which the original swarm was difficult to access. The second objective was to develop a trap that could be set out after swarm control, which would contain straggler bees. This would reduce problems caused by bees flying around the site, and would make the timing of a return visit much more flexible, or even make a return visit unnecessary.
Methods and results
Study site and bee swarms
We conducted this study in the Agricultural Operations area of the Experiment Station at UC Riverside, from July to September of 1994. The weather was always sunny during tests, which we conducted between 13:00 and 17:00. The bees were of mixed European races of Apis mellifera, probably predominantly A. mellifera ligustica.
We made artificial swarms from package bees, with about 7500 bees per swarm (Visscher et al. 1995). At four sites, we suspended the queen in a cage from a horizontal wooden crossbar fixed 1 m from the ground between two stakes, and shook the workers out to cluster around her. Within about 1 hour from establishment, some bees began to come and go from the swarm cluster in search of home sites.
At each site, we placed wooden crossbars and stakes, identical to those upon which the swarm was settled, approximately 5 m to either side of and in line with the swarm. We chose sites that were shady and which provided a relatively uniform distribution of visual landmarks. In all paired trials below, we determined which treatment we presented at which of the two crossbars of by a coin toss.
Straggler Bees
We left the swarm undisturbed for at least 4 hours to ensure that it had stabilized and that the bees had enough time to orient to their new site. We then removed the swarm by putting most of the bees and the queen back into the package cage, and removing it from the vicinity. The straggler bees consisted of bees that took flight during this process, as well as bees returning from scouting, foraging, or orientation flights. After removal of the swarm, we also removed the central crossbar upon which the swarm had rested. This prevented the stragglers from orienting visually to the crossbar or chemically to beeswax or other substances they had deposited while clustered.
Pheromone Trials
For these trials we presented two alternative lures, each hung from one of the remaining crossbars described above. The lures consisted of small blocks of plywood (25 x 50 x 6 mm) hung from wires through holes at one end. On each lure we spread test material over one surface of the block and allowed it to soak in for several seconds before presentation. About 1 h after swarm removal, and again the next day, 22 h after removal, we recorded whether most of the bees were one block or the other, or split between the blocks.
Queen Mandibular Pheromone
Synthetic queen mandibular pheromone (QMP) is a mixture of five components that are produced in the mandibular glands of queen honey bees, and elicits behavior from workers like that displayed toward queen bees (reviewed by Winston & Slessor 1992). It consists of 9-oxydecenoic acid, (-) and (+) isomers of 9 hydroxydecenoic acid, methyl-p-hydroxybenzoate, and 4-hydroxy-3-methoxy phenylethanol in a ratio of 118:50:22:10:1. One queen-equivalent (Qeq) of QMP is the amount found in the mandibular gland of an average honey bee queen.
Nasonov Pheromone
Synthetic Nasonov gland pheromone (NGP) is a mixture of components produced in the Nasonov gland of worker honey bees. Worker bees use Nasonov pheromone as an assembly pheromone. Our synthetic NGP consisted of citral and geraniol in a 2:1 ratio.
We tested 3 pairs of materials on the lures. To test whether QMP was attractive and would settle straggler bees, we tested 100 µl of solution of QMP in isopropanol (10 Qeq QMP /100 µl) versus 100 µl isopropanol. To test whether NGP was attractive alone, we tested 100 µl of 1:1 (v:v) mix of NGP and isopropanol versus 100 µl isopropanol. Since both of these were attractive, but QMP more so, we tested whether the addition of NGP to QMP increased the attraction over QMP alone, with 150µl of isopropanol-pheromone mixture containing 10 Qeq QMP / 150 µl and 37 µl NGP / 150 µl versus 150 µl of QMP alone in isopropanol (10 Qeq QMP/150µl)
For each of the above three trials, we performed tests on 8 swarms at 4 different sites. We analyzed the results of these tests statistically with a sign test (Daniel,1978).
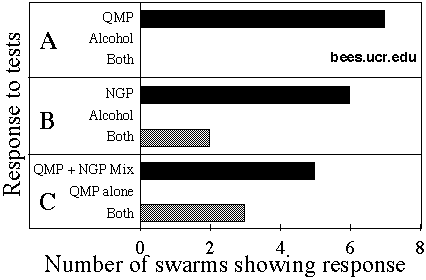
Fig. 1.Swarms presented with paired blocks containing pheromones, mixes of pheromones, or alcohol controls could go principally to one or the other block (named treatment) or large numbers of bees could go to each block (both), indicating no preference.
QMP attractiveness
QMP lures attracted significantly more bees in the swarm in every trial (P=0.008), tested against isopropanol control lures (Fig. 1 A). In one trial, all bees left the area, perhaps returning to the colony from which their package had been shaken, and this trial was omitted from analysis.
NGP attractiveness
NGP lures attracted most of the bees in 6 trials, and in 2 trials the cluster split between the NGP-treated blocks and the isopropanol-treated blocks (P=0.016; Fig. 1B). This effect was not as strong as the effect of QMP, above.
Mixed NGP + QMP versus QMP alone
Blocks treated with a mixture of QMP and NGP attracted bees significantly more often than those treated with QMP alone (P=0.031). However, this was a rather weak effect, since 5 of the swarms went nearly exclusively to the mix, but 3 split between the two baits (Fig. 1C).
Container trials
Trap design
Attractive baits could stabilize straggler bees, but a trap that would attract, retain, and kill stragglers would be even more useful. We designed a trap made from paper food buckets like those used for take-out at Chinese restaurants (1 liter size). These are waterproof, so they hold soap solutions, and have a wire bail from which to hang the trap. We bored 2 15-cm-diameter holes in opposite sides of each bucket, and stapled a cone of screening with an 8-mm-diameter hole in the end to the inside of the bucket over each hole. Bees enter these cones, and pass through the hole, but do not readily find the hole if they attempt to leave the trap. We stapled a cotton dental wick to one of the cover flaps of each bucket, and applied pheromone as above to these wicks. The construction of the traps is detailed in the appendix.
Repellency of insecticides
We conducted these trials to determine whether insecticidal soap or dichlorvos pesticide strips killed bees that entered the traps, and whether these insecticides were repellent to bees and interfered with the attractant. As before, we set up swarms and then removed them about 4 h later. We then presented two traps side by side on one crossbar. After 5 min we either counted the bees inside each trap, or weighed the trap with the bees on a precise portable electronic balance. We assayed bees from the same site repeatedly in this manner. To test whether such repeated tests were independent, we first presented a choice between two identical, empty traps with mixed pheromones. We then shook the bees out of the traps, and exchanged the right and left traps, to see if bees consistently chose the same trap of the pair.
Fig. 2.Test for independence of successive trials on empty traps, and tests for repellency of insecticides: dichlorvos-impregnated strips and 4% solutions of M-Pede® insecticidal soap.
We then performed similar manipulations, but this time with insecticides in one of the traps, and nothing in the other, to test for changes in attractiveness due to the insecticides. For these trials we used either 2.5 cm-square blocks of diclorvos-impregnated pest strips, or about 50 ml of a 4% solution of M-Pede® insecticidal soap. In both cases, the insecticides were beneath a screen held 2 cm above the base of the trap, so that bees could not contact the insecticide. In the case of dichlorvos this did not prevent toxicity, since it is a fumigant, but it did prevent toxicity in the insecticidal soap treatment.
We performed 11 tests on four different swarms and sites for insecticidal soap, and four tests on two swarms and sites for dichlorvos, and four tests on one swarm to test independence in repeated trials. We analyzed the results of these trials with ANOVA, with factors of swarm, treatment (empty or with insecticide) and upwind or downwind within a paired presentation.
As shown in Fig. 2, when given a choice between two empty traps baited with mixed pheromones, bees chose randomly between them (factor of trap had no significant effect in the ANOVA, F1,3 = 0.62, P=0.48). This suggests that chemicals deposited on the trap by the bees themselves do not enhance the attractiveness of the trap between closely spaced trials, so that repeated measurements on the same traps are independent.
Traps containing dichlorvos pesticide strips were quite repellent to bees (Fig 2; treatment F1,4=32.4, P=0.020). Bees were attracted to the traps by the pheromone, but then relatively few entered the entrances, often going part way in and then back out. Those which did go inside became quite agitated, and ran and flew inside the traps until they died. When only a dichlorvos-containing trap was presented, still few bees entered. When collected the next day, the trap contained only 5 bees (compared to hundreds for empty or soap-solution-containing traps). Most of the bees apparently either dispersed or died from pesticide exposure they received at the entrance or on the trap surface.
Traps containing soap solution were significantly less attractive to bees in paired tests with empty traps (Fig. 2; treatment F1,16 =6.0, P=0.026). This effect (with 2.4 as many bees entering the empty as the soap solution trap) was much weaker than that observed with dichlorvos insecticide (with 4.5 times as many bees entering the empty as the dichlorvos trap). Also, when we presented traps containing soap solution alone, all the bees usually entered the traps by the next morning (see below).
Evaluations of trapso evaluate how completely the traps developed in this study captured straggler bees, we placed traps at a site of swarm removal at approximately 1700 hours, with 200 ml of insecticidal soap solution (and no screen) in the bottom of the trap. We collected the traps at about 0900 the next morning, and counted any living bees on the outside or inside of the traps. We then estimated the total number of bees killed by draining the solution from the bees, weighing them, and weighing a subset of 10 bees. We also placed another trap, empty and with pheromone, at the site, to determine whether there were bees still in the area that had not entered the original trap. We checked these second traps in mid afternoon of the day it was placed.
Traps containing soap solution in most cases attracted all the straggler bees to enter. They collected an average of 403±105 (SD) bees in 9 trials. In five trials in which a backup trap was placed, only two collected any stragglers (2 and 3) the next day. In one case, following a particularly cool night, there were 26 (4.8%) of the bees on the outside of one of 2 traps tested over that night ; these may have entered had the trap been left in place until the temperature rose. Of the bees that entered traps, 98.9% ± 1.8% (mean ± SD, range 95.6% - 100%, N = 9) were killed; the remainder usually were hanging onto the wick inside the trap cover.
To quantify the effect of distance from the swarm clustering site on the trap entry rate of bees, we performed trials on four swarms at four sites in which we first measured the rate of arrival at a trap 5 m from the swarm site, then shook the bees out and measured the rate of arrival at a trap 35 m from the swarm site. We quantified bee arrivals by weighing the trap on a sensitive balance at one or two minute intervals.
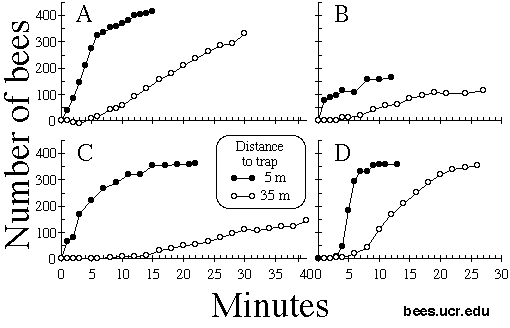
Fig. 3. Cumulative number of bees in or on traps placed 5 meters from swarm site (filled circles) or 35 meters from swarm site (open circles). Site B had a small number of straggler bees, site C had very little air movement.
Bees discovered and entered the traps most quickly when they were close to the original site of the swarm, but were able to find traps at least 35 m away, though the rate of bee arrival at 35 m was less than at 5 m (Fig. 3). Wind speed and direction influenced these rates: bees found traps upwind of the swarm site more quickly, and at 35 m, bees found traps more quickly when wind was blowing than when it was nearly still.
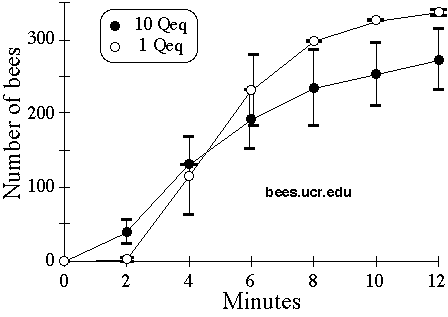
To determine whether smaller amounts of QMP could be effectively used, we compared the rate of arrival of bees at traps baited with a mixture of 1 Qeq QMP and 37 µl NGP against a mixture of 10 Qeq QMP and 37 µl NGP (as used above). We prepared straggler bees as usual, and measured their rate of arrival at a 1 Qeq trap placed 5 m from the swarm site. We then emptied the trap, and placed the closed cage containing the swarm and queen back at the swarm site, and allowed the stragglers to find and cluster on this cage for 1 hour. We then shook them off the cage, and measured their rate of arrival at a 10 Qeq trap placed 5 m from the swarm site in the opposite direction from the first trial. We repeated this only twice, and in both trials presented the 1 Qeq first, so the rates we measured on the two concentrations are not strictly comparable. However, Fig. 4 suggests that the attractiveness of 1 Qeq and 10 Qeq traps was similar. We also left 1 Qeq traps set up overnight 20 m from the swarm site; the number of stragglers captured and percentage alive was similar to the results above (324 and Fig 4. Mean ± SE (N=2) of cumulative number of bees in or on traps placed 5 meters from swarm site and baited with 37 µl NGP and either 1 Qeq QMP (open symbols) or 10 Qeq (filled symbols). Experimental conditions, rather than concentration differences, probably account for lower asymptote of 10 Qeq curve (see text) 253, with 98.5% and 100% dead).
Discussion
The "take-out" style trap is quite effective in realizing the objectives of this research. It attracts straggler bees after swarm removal; they enter it readily, and do not readily leave. If it is deployed with insecticidal soap solution, which persons doing swarm control are likely to have on hand, it traps and kills all or nearly all the straggler bees. The trap greatly reduces the possible interaction of people and straggler bees, and increases the flexibility of timing of swarm removal.
Both dichlorvos insecticide and insecticidal soap showed a significant repellency to bees. In the case of the dichlorvos, this was great enough to reduce the effectiveness of the trap greatly. With insecticidal soap, however, the repellency was smaller, and when traps containing insecticidal soap solution were presented alone, they attracted bees well, and were effective. Since the insecticidal soap worked well, is likely to be on hand when swarm abatement is performed, and poses fewer disposal problems due to its low toxicity, it will be the treatment of choice for most situations. If other insecticides were needed, it might be possible to find suitable insecticides with low repellency and high bee toxicity.
Our results suggest that 10 Qeq of QMP pheromone is more than is needed for effective trapping of straggler honey bees. QMP is by far the most expensive component of the traps. Since most of the testing was done with 10 Qeq trap baits, we are not confident in recommending 1 Qeq baits, but our results suggest that lower-dose baits deserve further evaluation, and may be fully adequate.
In use, our experience suggests that a baited trap should be hung as near to the swarm clustering site as practical, after the swarm cluster is destroyed. In situations where the swarm is difficult to access, or where it is desirable to attract bees a short distance away where they would pose less of a threat or attract less disturbance, the traps can be effective some distance away, but this should be kept as short as possible. If possible, the trap should be placed upwind. In our trial evaluations, the traps attracted bees away from the swarm clustering site, which is normally the most likely place for stragglers to reassemble (because of the odor of wax deposited by the clustered swarm, and the bees' spatial memory of the site). However, the traps might work faster and more reliably the branch or other site on which the swarm clustered is removed.
The traps should probably be removed the second or third day after deployment. This would allow stragglers that might be on the outside to move in. The traps effectively contain the insecticide solution for up to a week, though leakage might occur if the paper of the trap has been abraded or bent. When removing a trap, any bees inside but not killed can be killed effectively by covering the holes (or putting the trap in a plastic bag) and shaking the trap a few times to wet bees on the bait, cover, or walls.
The 1 l traps we used, with 200 ml of soap solution, trapped and killed up to 550 straggler bees, and could probably have held more. There is no data on the number of straggler bees in field swarm control situations. Our experience suggests these traps will usually contain all the stragglers from a careful and successful swarm destruction. However, more field experience is needed to evaluate whether one of these traps is sufficient, and whether multiple traps or larger traps would be effective in situations with large numbers of stragglers.
Our limited experience suggests that removal and disposal of the trap could be left to a homeowner or other client in many situations. If this were done, the client should be instructed when to remove it (2-3 days) and to check it first for bees on the outside, then place it in a bag and shake it, and dispose of the entire unit. Obviously, this option would need to be evaluated in individual situations, considering liability questions, the nature of the client, and relevant disposal regulations. Initially, at least, it would be best to have qualified personnel remove the traps, until sufficient experience confirms that they are sufficiently effective to permit safe handling by clients.
Besides their use with insecticide-killed swarms, these traps would probably be effective at capturing bees left behind when a swarm is captured and hived alive by a beekeeper, but not left in place until evening to pick up stragglers. They may also be effective in capturing straggler bees when bee colonies inside structures are hived or destroyed and the nest entrances sealed, and may reduce the need to perform structural bee colony control at night.
Acknowledgments
We are grateful to the California Mosquito and Vector Control Association for financial support of this research, and to Rick Vetter for assistance in the research, and improvements in the manuscript.
References cited
Daniel, W.W. 1978. Applied non parametric statistics. Houghton Mifflin. Boston, MA
Sames, W.J., W. T. Wilson, J. W. Smith, and H.D. Peterson. 1991. Effective destruction of honey bee colonies. Southwestern Entomologist 16:19-24.
Seeley, T.D., R.A. Morse, and P.K. Visscher. 1979. The natural history of the flight of honey bee swarms. Psyche 86:103-113.
Visscher, P.K.,. R.S. Vetter, and A.M. Khan. 1995. The use of insecticidal soap in abatement of honey bee swarms. Bee Science. in press
Winston, M.L. and K.N. Slessor. 1992. The essence of royalty: honey bee queen pheromone. American Scientist 80:374-385.
Figure legends
Appendix
Instructions and pictures for construction of traps
Trap components: prices and suppliers
| Item | Price/unit | Units/trap | ¢/trap | Supplier |
|---|---|---|---|---|
| Paper bucket | $8.00 /50 | 1 bucket | 16.0¢ | Smart & Final Iris Co. (Local) |
| Aluminum screening | $0.35/ft2 | 1/20 ft2 | 1.8¢ | Local hardware store |
| Dental wick | $15.70/5000 | 1 wick | 0.3¢ | Local dental supply company |
| Citral | $8.30/5O ml | 1/50 ml | 0.3¢ | Sigma |
| Geraniol | $11.50/25 ml | 1/50 ml | 0.9¢ | Sigma |
| QMP | $95.00/1185 Qeq | 10 Qeq | 80.2¢ | Phero-Tech |
| Total (10 Qeq QMP) | 99.5¢ | |||
| QMP | $95.00/1185 Qeq | (1 Qeq?) | 8.0¢ | |
| Total for 1 Qeq QMP | 27.4¢ |
Sigma Chemical Co. P.O. Box 14508, St. Louis, MO 63178. (800)-325-3010
Phero-Tech Inc. 7572 Progress Way, Delta, V4G1E9 BC, Canada. (800) 665-0076